Embracing continuous manufacturing: revolutionizing pharmaceutical production
In today’s rapidly evolving pharmaceutical landscape, the need for innovative and efficient manufacturing processes has become paramount. Traditional batch manufacturing methods, although effective, have limitations that hinder agility, productivity, and cost-effectiveness.
However, a groundbreaking solution has emerged to address these challenges: continuous manufacturing.
In this blog post, we will explore the transformative power of continuous manufacturing in the pharmaceutical industry and its potential to revolutionize drug production. Moreover, we will introduce how our partner IMA implements continuous manufacturing into their production lines.
Understanding Continuous Manufacturing
Continuous manufacturing in the pharmaceutical industry refers to a streamlined, uninterrupted process where raw materials are continuously fed into a system and transformed into finished products in a continuous flow.
Unlike batch manufacturing, which involves discrete steps and requires frequent start-up and shutdown, continuous manufacturing enables a seamless production cycle with real-time monitoring and control.
Advantages of Continuous Manufacturing
- Enhanced Efficiency: Continuous manufacturing eliminates the need for intermediate storage, reducing handling and associated risks. This leads to optimized production timelines, increased equipment utilization, and minimized material waste, ultimately improving efficiency and productivity.
- Superior Quality: The continuous nature of the manufacturing process allows for real-time monitoring and quality control. By continuously analyzing critical quality attributes, manufacturers can promptly identify and rectify any deviations, ensuring consistent product quality throughout the production cycle.
- Flexibility and Scalability: Continuous manufacturing offers greater flexibility in adjusting production volumes and formulations, allowing manufacturers to respond rapidly to market demands. Additionally, it facilitates seamless scale-up or scale-down operations, enabling efficient technology transfers and reducing time-to-market.
- Cost Savings: With its efficient resource utilization, reduced downtime, and minimized waste, continuous manufacturing has the potential to deliver significant cost savings over traditional batch processes. The streamlined production cycle also eliminates the need for large storage facilities, reducing overhead costs associated with inventory management.
Continuous Manufacturing in Action
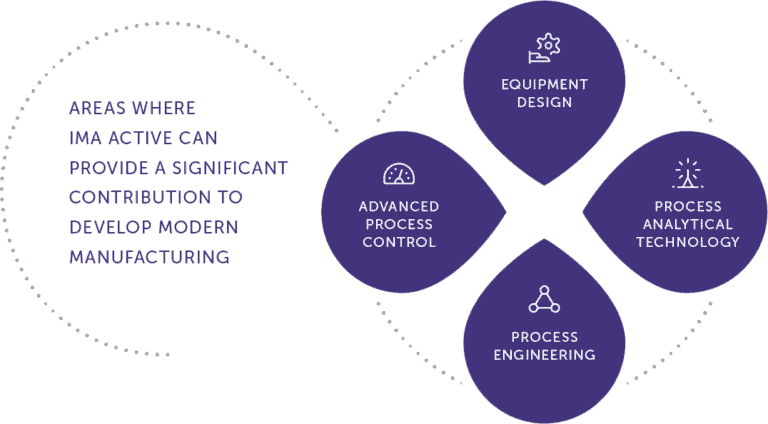
To truly grasp the impact of continuous manufacturing, let’s explore how IMA is spearheading this transformation. IMA’s continuous manufacturing solutions offer a comprehensive range of integrated technologies that streamline the entire pharmaceutical production process.
Key Technologies and Features
- Mixing and Blending: IMA’s continuous manufacturing solutions incorporate advanced mixing and blending technologies that ensure consistent and homogenous drug formulations, minimizing variability and optimizing product quality.
- Granulation and Drying: Continuous granulation and drying systems enable precise control over particle size, uniformity, and moisture content, resulting in improved product stability and dissolution properties.
- Tableting and Coating: IMA’s cutting-edge tableting and coating technologies guarantee precise dosing and uniform coating application. These systems offer flexibility in tablet design and allow for the incorporation of multiple active ingredients.
- Real-time Monitoring and Control: IMA’s continuous manufacturing solutions integrate robust monitoring and control systems that provide comprehensive data on critical process parameters, facilitating continuous process optimization and adherence to regulatory requirements.
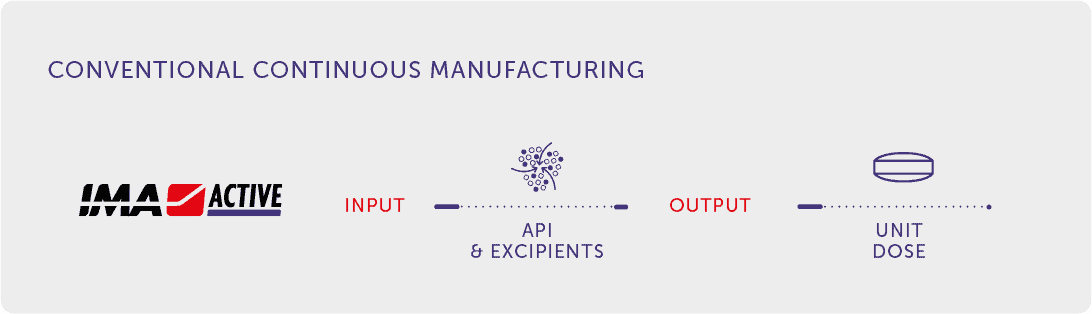
Conventional Continuous Manufacturing
On the other R&D front IMA Active works on continuous processes by revisiting current technologies, embracing a concept of Continuous Manufacturing more closely related to conventional solid forms. This initiative aims primarily to introduce continuous equipment into the market that can be integrated with conventional technologies, even in an existing plant, in order to improve the production performance of existing drugs.
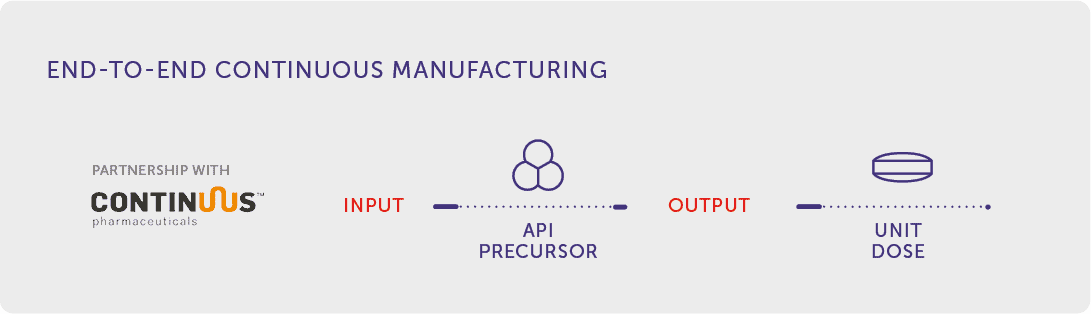
End-to-End Continuous Manufacturing
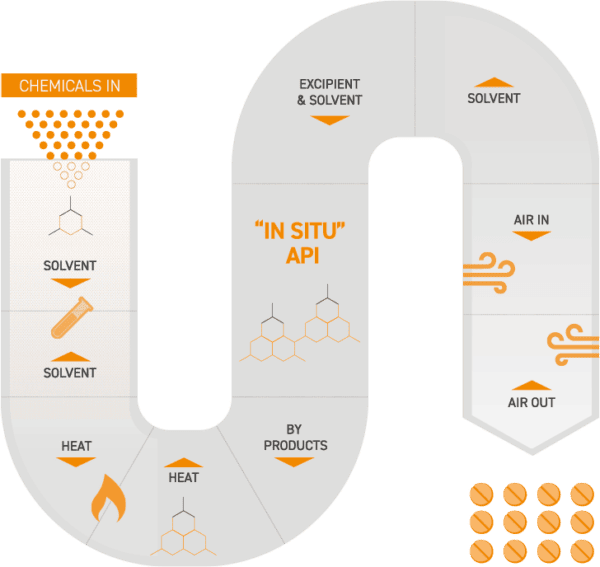
A more disruptive front of IMA Active R&D uses a new production platform called Integrated Continuous Manufacturing (ICM), which enables seamless end-to-end Continuous Manufacturing processes that incorporate API formation steps with final drug product formulation.
The Road Ahead: Incorporating Continuous Manufacturing
Pharmaceutical production is undergoing a paradigm shift, and implementing continuous manufacturing holds immense potential for stakeholders across the industry. Decision-makers in pharmaceutical companies must recognize the benefits of this transformative approach and invest in technologies and infrastructure that support its implementation.
Key stakeholders, including regulatory bodies, must collaborate with manufacturers to establish clear guidelines and standards for continuous manufacturing. This collaboration will foster innovation, ensure product quality and safety, and expedite the adoption of continuous manufacturing on a broader scale.
Continuous manufacturing is revolutionizing the pharmaceutical industry, redefining the way drugs are produced. With its efficiency, superior quality control, flexibility, and cost-saving potential, continuous manufacturing is ready to become the industry standard.
Reach out to us if you want to know more about continuous manufacturing and the opportunities for your production.
You can find our contact details here or fill out the form below.